

Cryo-electron microscopy (cryo-EM) was used to study monoclonal antibodies (mAbs) that modulate Peptidylarginine deiminase 4 (PAD4), an enzyme implicated in autoimmune disease. Structures of activating and inhibitory Fab–PAD4 complexes revealed how antibody binding at allosteric sites affected enzyme activity by stabilizing distinct conformations. This review highlights the major findings of the paper “Antibody discovery identifies regulatory mechanisms of protein arginine deiminase 4” (1), and highlights how cryo-EM epitope mapping can guide lead antibody selection, differentiating functionally superior mAbs based on structural mechanism, not just binding affinity.
Cryo-EM has emerged as a critical tool in structure-based antibody discovery, allowing direct visualization of antigen–antibody complexes. It excels in mapping conformational epitopes, resolving functional differences between antibody candidates, and elucidating allosteric mechanisms. For enzymatic targets like PAD4, which requires calcium and oligomerization for activity, cryo-EM can show how antibodies influence these features to promote or suppress activity.
Peptidylarginine deiminase 4 (PAD4) catalyzes the citrullination of arginine residues in histones, a process that contributes to autoimmunity and inflammation. Its activity is calcium-dependent and requires dimerization to be catalytically optimal. Small-molecule inhibitors have shown promise in preclinical models, but often lack selectivity due to conserved active sites across the PAD family. Allosteric modulation through antibodies presents a more selective strategy. Recent work by Zhou et al. (2024) used cryo-EM to reveal how different Fabs modulate PAD4 activity by binding at structurally distinct allosteric sites.
Two lead antibody candidates were selected for structural investigation: hA362, a functional activator of PAD4, and hI365, a selective allosteric inhibitor. Both antibodies exhibit nanomolar binding affinity and target non-overlapping epitopes on the PAD4 N-terminal domain. Cryo-EM reconstructions at near-atomic resolution captured these Fab–antigen complexes in distinct functional states—hA362 stabilizing an active dimeric conformation, and hI365 locking the enzyme in an inactive, Ca2+-bound state.
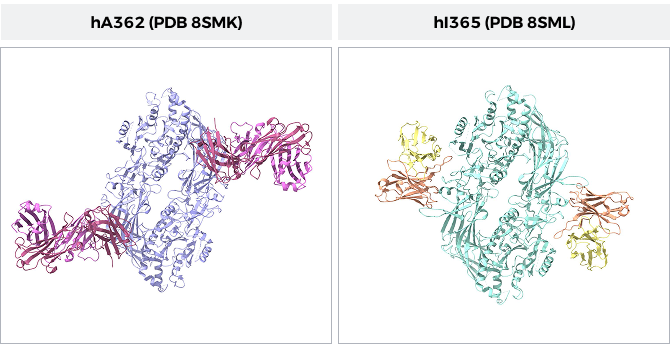
Figure 1: Comparison of the two PAD4 structures with bound Fabs. Left: hA362 (red and pink) bound to PAD4 (purple). Right: hI365 (yellow and orange) bound to PAD4 (cyan). Image created in Chimera X from PDB: 8SMK and 8SML.
Cryo-EM at 3.5 Å resolution revealed that the hA362 Fab binds across the dimer interface of PAD4, stabilizing its active dimeric form. The Fab primarily contacts the N-terminal domain of one PAD4 monomer but also interacts with the interface loop of the adjacent subunit. This bridging contact reinforces an intermolecular salt bridge (e.g., between R441 and D465), helping to lock the active-site loops in a catalytically competent conformation (Figure 2). As a result, hA362 binding enhances PAD4 enzymatic activity by promoting and stabilizing its active state.
Structural analysis showed that the heavy-chain complementarity-determining regions (CDRs) of hA362 engage both monomers, creating a unique dimer-stabilizing architecture. Mutation studies confirmed the importance of interface residues: altering PAD4 Y435 disrupted binding and activation. These results underscore how the antibody acts not by binding the active site, but by enforcing the correct oligomeric state and conformational arrangement of active site loops.
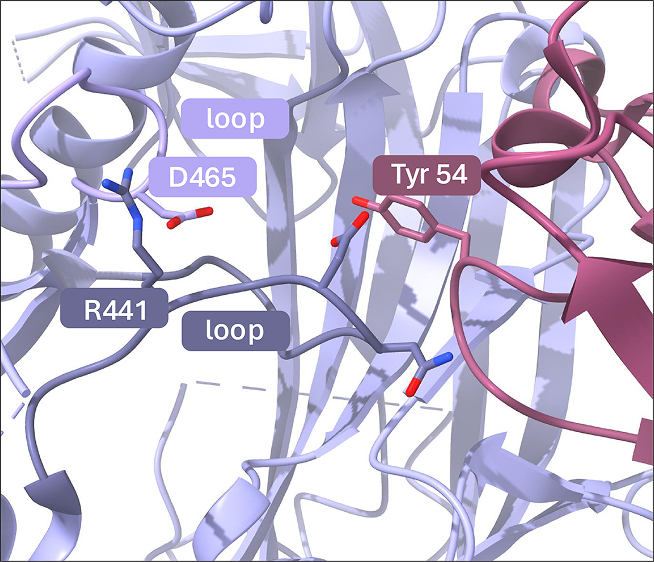
Figure 2: The hA362 Fab activates PAD4 by locking active-site loops (S-loop and L-loop) in a catalytically competent conformation. R441 and D465 reinforce a intermolecular salt bridge.
Conversely, the inhibitory Fab hI365 binds monomeric and dimeric PAD4 and disrupts calcium-induced activation. Cryo-EM at 3.3 Å resolution showed hI365 engaging a calcium-binding site on the N-terminal domain. It inserts a loop into the calcium pocket, displacing essential residues required for Ca2+ coordination. This prevents the conformational change necessary for PAD4 to transition into an active state, effectively locking the enzyme in an inactive conformation.
Importantly, hI365 binds selectively to the Ca2+-bound form of PAD4, enabling it to target the enzyme specifically under activation conditions. This makes it particularly relevant for therapeutic intervention in inflamed tissues, where calcium concentrations are elevated.
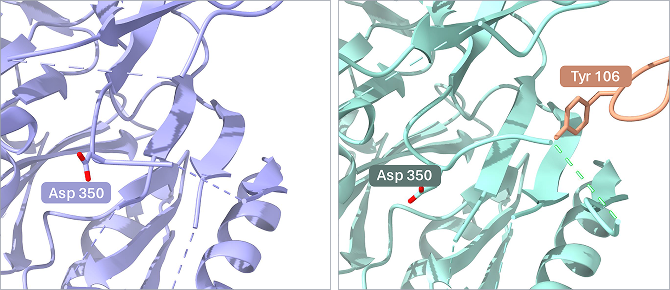
Figure 3. Tyrosine 106 interacts with a loop, disrupting calcium binding. Left: PAD4 bound to hA362, leading to an activated state. Left: PAD4 bound to hI365 leading to an inhibited state due to the Fab (Tyr 106) interacting with the loop (Asp 350), displacing it into the calcium binding pocket.
The two antibodies bind at non-overlapping allosteric sites, each altering PAD4’s function via a distinct mechanism: hA362 promotes dimerization and activity; hI365 prevents calcium-induced activation. Structural insights from cryo-EM revealed the specific conformational states and interaction mechanisms responsible for activity modulation.
Cryo-EM enabled:
Information such as this is essential for prioritizing lead candidates. For instance, hI365’s ability to bind the active (Ca2+-bound) form of PAD4 and lock it in an inactive conformation makes it a strong therapeutic candidate. Its epitope is highly specific and avoids the conserved active site, potentially reducing off-target effects. Structural comparisons demonstrated that this epitope does not overlap with other PAD isoforms, supporting selectivity.
Similarly, hA362 offers a tool for probing PAD4 function by selectively enhancing its activity. In diseases where PAD4 may be underactive, such as in certain cancer models, such antibodies could have therapeutic or investigative applications. Structural knowledge guided the optimization of these antibodies, including removing non-essential hydrophobic residues that contributed to aggregation.
This review exemplifies how cryo-EM can de-risk and accelerate antibody development:
In the PAD4 example, cryo-EM clarified subtle yet critical differences between Fab candidates. By visualizing how Fabs interact with their epitopes and alters structure and activity, researchers can select the most therapeutically promising inhibitor/activator for further development.
Mechanism-based antibody discovery depends on accurate structural understanding. Cryo-EM enables this by resolving Fab–antigen complexes at near-atomic resolution. In this paper, cryo-EM showed how different Fabs induced distinct allosteric effects—some activating, others inhibitory—through specific conformational stabilization or disruption.
Such structural data informs both candidate selection and rational antibody engineering, particularly for conformationally regulated enzymes like PAD4. For pharmaceutical developers, it may provide the clarity needed to move from a pool of binders to a focused lead with validated mechanism, specificity, and therapeutic potential.
Ultimately, cryo-EM bridges the gap between biochemical function and molecular mechanism. By directly comparing Fab–antigen complexes, developers can select the most promising therapeutic candidate not only based on binding affinity or potency but on verified structural action at the molecular level—a key step toward reducing development risk and enhancing success in clinical translation. Antibody discovery depends on accurate structural understanding, and cryo-EM enables this by resolving Fab–antigen complexes at near-atomic resolution. Cryo-EM showed how different Fabs induced distinct allosteric effects—activating and inhibitory—through specific conformational stabilization or disruption.
1 Zhou, Xin, Sophie Kong, Allison Maker, Soumya G. Remesh, Kevin K. Leung, Kliment A. Verba, and James A. Wells. “Antibody Discovery Identifies Regulatory Mechanisms of Protein Arginine Deiminase 4.” Nature Chemical Biology 20, no. 6 (June 2024): 742–50. https://doi.org/10.1038/s41589-023-01535-8.

You are currently viewing a placeholder content from Vimeo. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationYou are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationYou need to load content from reCAPTCHA to submit the form. Please note that doing so will share data with third-party providers.
More Information