High-quality results accessible and timely for your needs with our ability to process hundreds of samples each month.
Experience the first AI solution to quantify LNP morphology at >98% precision and human like accuracy.
Our team of experts is the backbone of our GMP-certified operations, ensuring the highest levels of quality and compliance.
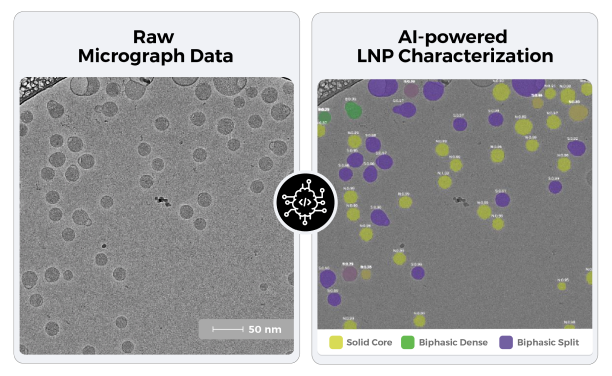
At ATEM we have developed a machine learning based algorithm to transform cryo-EM LNP images into quantitative morphological insights.
“Automation is the key
to make nano-imaging
data quantitative”

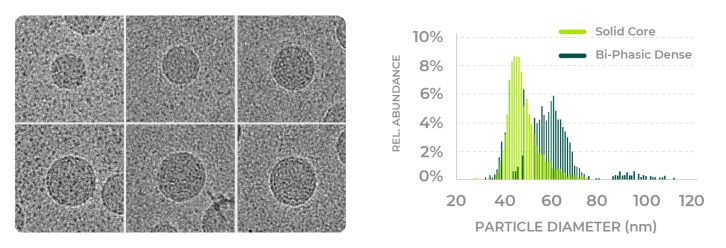
ATEM’s machine learning algorithm can characterize the size and size distribution of the LNP at single particle precision.
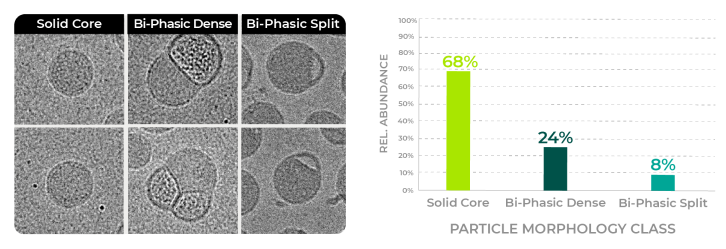
The machine learning model classifies the particles into the main observed particle morphologies of Solid Core, Biphasic Split and Biphasic Dense (Blebbed) particles.
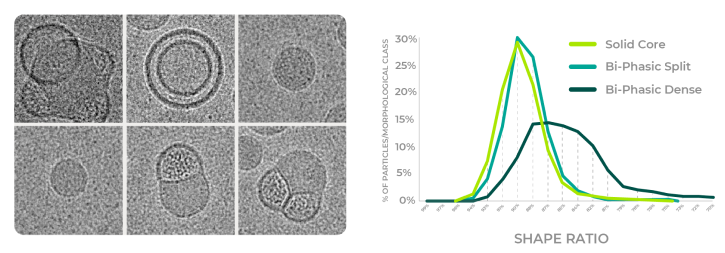
The Aspect Ratio (Shape Ratio) identifies the shape of the LNP, relative to a perfect sphere. Cryo-EM is the only method to provide this level of insights.
Based on a random draw study we can see that only 2.000+ particles the statistical significance becomes robust. This is important when drawing conclusions about less frequently present morphological classes.
< 1.0 nm
95% Confidence Interval
(Avg. Particle Diameter, 5000 particles)
Based on technical replicates of the same sample have been analyzed. And the result shows a Standard Deviation of sub 1 nm across all replicates. Additional information can be obtained from the application note.
< 1.0 nm
Standard Deviation
(Avg. Particle Diameter, 5000 particles)
Based on benchmarking the results of a trained human operator against the results generated by the machine learning model show human like results (>96% accuracy). Technical replicates demonstrate a 1-2% standard deviation on classification accuracy.
96.3%
⌀ Classification Accuracy
(Particle Morphological Class)
We are dedicated to providing an exceptional service, while our scientists guide you through the entire process
Gain valuable insights to decipher the in-depth characteristics of your most promising formulations or obtain previously inaccessible perspectives on challenges.
Better understand bottlenecks in your scale-up or downstream processes and their effect on LNP drug product. Gain the ability to more effectively address and mitigate them.
Ensure the most advanced understanding into batch-to-batch consistency to ensure your processes and products always control for highest LNP drug product integrity.
Extend your IND filing with quantitative LNP cryo-EM data to showcase product quality, production consistency and in-depth understanding of its characteristics.
We recognize that every client has unique needs and preferences. Explore our packages’ features and choose the one that best fits your requirements.
Best for early R&D & exploratory studies
Best for process optimization & comparability studies
Best for critical quality assesments & regulatory submissions
Note: The mandatory field is marked. All other fields are voluntary. Your data will only be collected and stored electronically for the strict purpose of processing and responding to your request. Information on data processing can be found here: Privacy Policy.
You need to load content from reCAPTCHA to submit the form. Please note that doing so will share data with third-party providers.
More Information